 J-Plasma from Bovie Medical Corporation is a patented plasma-based med spa procedure to resurface the skin and rejuvenate soft tissue in specific areas.
J-Plasma makes use of an innovative helium ionization process to create a focused and stable plasma beam that allows the treatment provider to resurface the skin with higher precision and in a minimally invasive way.
The advanced J-Plasma device can deliver three separate energy modalities to match with the skin tightening and resurfacing needs of different patients. Rejuvenate Medical Spa, led by Dr. Bijan Farah, provides J-Plasma treatment to patients in Encino, Sherman Oaks, San Fernando, Calabasas, Woodland Hills, Tarzana, Los Angeles, CA, and other cities and neighborhoods in this region of California.
J-Plasma from Bovie Medical Corporation is a patented plasma-based med spa procedure to resurface the skin and rejuvenate soft tissue in specific areas.
J-Plasma makes use of an innovative helium ionization process to create a focused and stable plasma beam that allows the treatment provider to resurface the skin with higher precision and in a minimally invasive way.
The advanced J-Plasma device can deliver three separate energy modalities to match with the skin tightening and resurfacing needs of different patients. Rejuvenate Medical Spa, led by Dr. Bijan Farah, provides J-Plasma treatment to patients in Encino, Sherman Oaks, San Fernando, Calabasas, Woodland Hills, Tarzana, Los Angeles, CA, and other cities and neighborhoods in this region of California.
J-Plasma Enrollment in Clinical Study
Bovie Medical Corporation, the developer of J-Plasma, has now announced that it has enrolled the first patient in an IDE (Investigational Device Exemption) clinical study in the US. This study will reveal more facts about the efficacy and safety of J-Plasma technology for dermal skin resurfacing. This comprehensive clinical study will be carried out at multiple centers, and will evaluate the benefits of J-Plasma procedure for the diminishing of facial wrinkles, creases and rhytides. The study will enroll 55 eligible patients and will be performed at up to five investigational centers. Enrolled patients will go through one treatment with the J-Plasma technique. Their severity of facial wrinkles will be assessed at baseline and thereafter at every follow up point. According to Charlie Goodwin, CEO of Bovie Medical, the developer’s long-term clinical strategy aims to expand the clinical indications of J-Plasma for use in aesthetic enhancement, starting with dermal skin resurfacing procedures. With the enrollment of the first patient in the skin resurfacing clinical study, the company has now achieved an important milestone in this journey. Bovie Medical will utilize the results of this study to demonstrate the aesthetic benefits and safety of J-Plasma and submit the technology to the US FDA for their review and clearance.Why choose J-Plasma dermal skin resurfacing?
Patients choose J-Plasma med spa procedure for skin rejuvenation due to the following reasons:- Improved results achieved in a minimally invasive way
- Comfortable in-office procedure
- Can be completed in about one hour
- Performed using only local anesthesia
- Almost no visible incisions or scarring
 In recent years, CoolSculpting has emerged as one of the most effective and reliable non-invasive fat reduction treatments available for women as well as men.
In recent years, CoolSculpting has emerged as one of the most effective and reliable non-invasive fat reduction treatments available for women as well as men. 

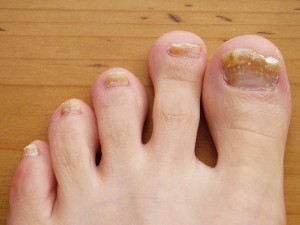 Onychomycosis or nail fungus is a common condition that typically appears as a yellow or white spot underneath the tip of a fingernail or toenail.
In absence of timely treatment, the fungal infection can spread deep into the nail, creating conspicuous aesthetic concerns. Topical or oral anti-fungal medications may only provide temporary relief, and the nail fungus may recur.
Laser treatment at a qualified med spa can offer an advanced way to get rid of nail fungus with minimal risk of recurrence. The procedure is safe and effective with long-term results. However, to ensure success, the laser treatment should only be performed by an expert laser technician under the supervision of an experienced physician.
Pillar of the community Rejuvenate Medical Spa, led by Dr. Bijan Farah, provides advanced laser based solutions to treat nail fungus for patients in Encino, Sherman Oaks, San Fernando, Calabasas, Woodland Hills, Tarzana, Los Angeles, CA, and surrounding locations in this region of SoCal.
Onychomycosis or nail fungus is a common condition that typically appears as a yellow or white spot underneath the tip of a fingernail or toenail.
In absence of timely treatment, the fungal infection can spread deep into the nail, creating conspicuous aesthetic concerns. Topical or oral anti-fungal medications may only provide temporary relief, and the nail fungus may recur.
Laser treatment at a qualified med spa can offer an advanced way to get rid of nail fungus with minimal risk of recurrence. The procedure is safe and effective with long-term results. However, to ensure success, the laser treatment should only be performed by an expert laser technician under the supervision of an experienced physician.
Pillar of the community Rejuvenate Medical Spa, led by Dr. Bijan Farah, provides advanced laser based solutions to treat nail fungus for patients in Encino, Sherman Oaks, San Fernando, Calabasas, Woodland Hills, Tarzana, Los Angeles, CA, and surrounding locations in this region of SoCal.
 Childbirth, aging, heredity, and other factors can sometimes create muscle and tissue laxity in the vaginal canal.
If a woman is bothered by the lax appearance of the vagina due to weak tissue, she may choose an advanced non-surgical med spa vaginal rejuvenation procedure called ThermiVa. With this safe and proven treatment, it is possible to restore the pre-pregnancy appearance and feel of the vagina.
ThermiVa vaginal tightening procedure can be performed in an office setting, and does not involve any cuts, incisions, injections or medications. Rejuvenate Medical Spa, led by Dr. Bijan Farah, provides ThermiVa vaginal rejuvenation to patients in Encino, Sherman Oaks, San Fernando, Calabasas, Woodland Hills, Tarzana, Los Angeles, CA, and surrounding communities.
Childbirth, aging, heredity, and other factors can sometimes create muscle and tissue laxity in the vaginal canal.
If a woman is bothered by the lax appearance of the vagina due to weak tissue, she may choose an advanced non-surgical med spa vaginal rejuvenation procedure called ThermiVa. With this safe and proven treatment, it is possible to restore the pre-pregnancy appearance and feel of the vagina.
ThermiVa vaginal tightening procedure can be performed in an office setting, and does not involve any cuts, incisions, injections or medications. Rejuvenate Medical Spa, led by Dr. Bijan Farah, provides ThermiVa vaginal rejuvenation to patients in Encino, Sherman Oaks, San Fernando, Calabasas, Woodland Hills, Tarzana, Los Angeles, CA, and surrounding communities.


 Hair reduction and hair loss are a common concern for both men and women in the US today. Although hair transplantation is available to restore hair loss, but many people want to avoid an invasive procedure. For all such candidates, Rejuvenate
Hair reduction and hair loss are a common concern for both men and women in the US today. Although hair transplantation is available to restore hair loss, but many people want to avoid an invasive procedure. For all such candidates, Rejuvenate 